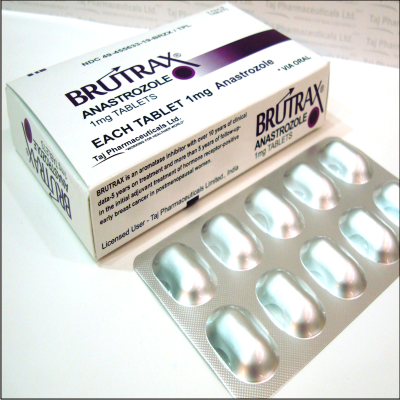
BRUTRAX®
Anastrozole 1mg Tablets
 |
 |
|
The Taj Pharmaceuticals Limited (Taj
Group) has operations in
every major international market
|
|
|
|
|
Contact us |
Address: |
|
BRUTRAX® (anastrozole) Tablets BRUTRAX® (anastrozole 1mg Tablets)Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international double-blind randomised placebo-controlled trial Anastrozole is used to treat breast cancer in women who have already gone through menopause and no longer have menstrual periods (postmenopausal women ) Anastrozole (INN) (marketed under the trade name Brutrax by AstraZeneca) is a non-steroidal aromatase-inhibiting drug approved for treatment of breast Consumer information about the medication ANASTROZOLE - ORAL (Brutrax) includes side effects drug interactions recommended dosages and storage Official Web site for BRUTRAX® (anastrozole) Tablets Learn about an early breast cancer treatment option breast cancer treatment coping strategies nutrition Learn about the prescription medication Brutrax (Anastrozole) Get information about a hormonal therapy called Anastrozole (Brutrax ®) which is used to treat breast cancer Physician reviewed anastrozole patient information - includes anastrozole description dosage and directions Anastrozole is used to treat breast cancer in women who have already gone through menopause and no longer have menstrual periods Anastrozole Sandoz is a non-steroidal aromatase inhibitor which reduces the amount of oestrogen (female sex hormone) made by the body in postmenopausal Anastrozole medical facts from brutrax com A new way to prevent breast cancer: anastrozole - Cancer Research BRUTRAX anastrozole 1 mg breast cancer treatment coping nutrition exercise community support side effects dosage prescribing information anastrozole oral Brutrax anastrozole oral effectiveness satisfaction ease of use medication medications medicine drug drugs prescription drugs user ratings drug ratings drug reviews rate a drug treatment side effects drug interactions drug information medical information medical advice warnings overdose drug images over the counter indications precautions Brutrax Anastrozole drug description chemical structure black box warning medications drugs generic fda approved patient labeling reviews professionals clinicians clinical anastrozole directions dose anastrozole Brutrax breast cancer aromatase inhibitor hormones hormone treatment side effects therapy hormonal Brutrax hormone antagonist aromatase inhibitor postmenopausal breast anastrozole tablets manufacturer anastrozole tablets supplier anastrozole export import anastrozole Generic anastrozole tablets manufacturer anastrozole tablets anastrozole tablets price manufacturing possibilities medicine health uses anastrozole trials anastrozole manufacturing anastrozole India trading anastrozole product anastrozole supplier supple anastrozole suppliers anastrozole vendor anastrozole vendors price anastrozole prices
|
Frequency Asked Questions

What is the most important information I should know about BRUTRAX®?
BRUTRAX® may cause serious side effects including: heart disease. Women with early breast cancer, who have a history of blockage in their heart arteries (ischemic heart disease) and who take BRUTRAX®, may have an increase in symptoms of decreased blood flow to their heart compared to similar women who take tamoxifen.
Get medical help right away if you have new or worsening chest pain or shortness of breath during treatment with BRUTRAX®.
What is BRUTRAX®?
BRUTRAX® is a prescription medicine used in women after menopause (“the change of life”) for:
⇒ treatment of early breast cancer
♦ after surgery
♦ in women whose breast cancer is hormone receptor-positive
⇒ the first treatment of breast cancer that has spread to nearby tissue or lymph nodes (locally advanced) or has spread to other parts of the body (metastatic), in women whose breast cancer is hormone receptor-positive or the hormone receptors are not known.
⇒ treatment of advanced breast cancer, if the cancer has grown, or the disease has spread after tamoxifen therapy BRUTRAX® does not work in women with
breast cancer who have not gone through menopause (premenopausal women).
Who should not take BRUTRAX®?
Do not take BRUTRAX® if you:
• Are pregnant or able to become pregnant. BRUTRAX® may harm your unborn baby. If you become pregnant while taking BRUTRAX®, tell your doctor right away.
• Have not gone through menopause (are premenopausal).
• Have had severe allergic reaction to anastrozole or any of the ingredients in BRUTRAX®. See the end of this leaflet for a complete list of ingredients in BRUTRAX®. Symptoms of a severe allergic reaction to BRUTRAX® include: swelling of the face, lips, tongue or throat, trouble breathing or swallowing, hives and itching.
What should I tell my doctor before taking BRUTRAX®?
Before you take BRUTRAX®, tell your doctor if you:
- Have not gone through menopause. Talk to your doctor if you are not sure.
- Have or had a heart problem.
- Have been told you have bone thinning or weakness (osteoporosis)
- Have high cholesterol
- Have any other medical conditions
- Are pregnant or plan to become pregnant. BRUTRAX® may harm your unborn baby. See “who should not take BRUTRAX®?”
- Are breastfeeding or plan to breastfeed. It is not known if BRUTRAX® passes into breast milk. You and your doctor should decide if you will take BRUTRAX® or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take:
- You should not take BRUTRAX® if you take tamoxifen. Taking BRUTRAX® with tamoxifen may lower the amount of BRUTRAX® in your blood and may cause BRUTRAX® not to work as well.
- Medicines that contain estrogen. BRUTRAX® may not work if taken with any of these medicines:
 hormone replacement therapy
hormone replacement therapy birth control pills
birth control pills estrogen creams
estrogen creams vaginal rings
vaginal rings vaginal suppositories
vaginal suppositories
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take BRUTRAX®?
- Take BRUTRAX® exactly as your doctor tells you to take it.
- Continue taking BRUTRAX® until your doctor tells you to stop.
- BRUTRAX® can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next does, skip the missed dose. Take your next regularly scheduled dose. Do not take two doses at the same time.
- If you take too much BRUTRAX®, call your doctor or go to the nearest hospital emergency room right away.
If you take too much ARMIDEX, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of BRUTRAX?
BRUTRAX may cause serious side effects including:
- See “What is the most important information I should know about BRUTRAX?”
- bone thinning or weakness (osteoporosis). BRUTRAX lowers estrogen in your body, which may cause your bones to become thinner and weaker. This may increase your risk of fractures, especially of your spine, hip and wrist. Your doctor may order a bone mineral density test before you start and during treatment with BRUTRAX to check you for bone changes.
- increased blood cholesterol (fat in the blood). Your doctor may do blood tests to check your cholesterol while you are taking BRUTRAX.
- skin reactions. Stop taking BRUTRAX and call your doctor right away if you get any skin lesions, ulcers, or blisters.
- severe allergic reactions. Get medical help right away if you get:
 swelling of your face, lips, tongue, or throat
swelling of your face, lips, tongue, or throat trouble swallowing or breathing
trouble swallowing or breathing
- Stop taking BRUTRAX and call your doctor right away if you have any of these signs or symptoms of a liver problem:
 a general feeling of not being well
a general feeling of not being well yellowing of your skin or whites of your eyes
yellowing of your skin or whites of your eyes pain on the right side of your stomach-area (abdomen)
pain on the right side of your stomach-area (abdomen)
Common side effects in women taking BRUTRAX include:
 hot flashes
hot flashes weakness
weakness joint aches
joint aches joint pain, stiffness or swelling (arthritis)
joint pain, stiffness or swelling (arthritis) pain
pain sore throat
sore throat high blood pressure
high blood pressure depression
depression-
 nausea and vomiting
nausea and vomiting -
 rash
rash  back pain
back pain sleep problems
sleep problems bone pain
bone pain headache
headache swelling of your legs, ankles, or feet
swelling of your legs, ankles, or feet increased cough
increased cough shortness of breath
shortness of breath build up of lymph fluid in the tissues of your affected arm (lymphedema)
build up of lymph fluid in the tissues of your affected arm (lymphedema)
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of BRUTRAX. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BRUTRAX?
Store BRUTRAX at room temperature between 68°F to 77°F (20°C to 25°C).
Keep BRUTRAX and all medicines out of the reach of children.
General information about the safe and effective use of BRUTRAX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not take BRUTRAX for a condition for which it was not prescribed. Do not give ARMIDEX to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about BRUTRAX that is written for health professionals. For more information call 1-866-992-9276 or go to www.BRUTRAX.com.
What are the ingredients in BRUTRAX?
Active ingredient: anastrozole
Inactive ingredients: lactose, magnesium stearate, hydroxypropylmethylcellulose, polyethylene glycol, povidone, sodium starch glycolate, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
May/2013
Distributed by:
Taj Pharma India Brands
Taj Pharma Group (India)
P.O. Box No. 120002,
Azad Nagar Post office,
Andheri (w), Mumbai - 400 053.
India.
© Copyright 2019 Taj Pharmaceuticals Limited. All rights reserved.
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 1 mg
BRUTRAX®
anastrozole tablets
1 mg tablets
Rx only
Taj Pharma India Brands
Taj Pharma Group (India)
P.O. Box No. 120002,
Azad Nagar Post office,
Andheri (w), Mumbai - 400 053.
India.
VISION
 At Taj Pharmaceuticals our goal is to create innovative drugs and diagnostic tools for the medicine of tomorrow. Rapid progress in the sciences is providing crucial insights into the underlying molecular mechanisms of disease, opening up new ways for prevention, diagnosis and therapy. With the expertise in Diagnostics and Pharmaceuticals, Taj Pharmaceuticals is best placed to capture the value from the Healthcare Revolution.
At Taj Pharmaceuticals our goal is to create innovative drugs and diagnostic tools for the medicine of tomorrow. Rapid progress in the sciences is providing crucial insights into the underlying molecular mechanisms of disease, opening up new ways for prevention, diagnosis and therapy. With the expertise in Diagnostics and Pharmaceuticals, Taj Pharmaceuticals is best placed to capture the value from the Healthcare Revolution.
The future will be shaped by fundamental paradigm shifts ushering in a new era of more effective medicine. Diagnosis, prevention and treatment will be linked more closely than ever before in integrated strategies offering patients and healthcare providers highly focused, individualised care. We are confident that, with our outstanding workforce, cutting-edge science and state-of-the-art technology base, we can meet tomorrow's needs for integrated healthcare solutions.
MISSION

Taj Pharmaceuticals is committed to a system of corporate governance that conforms to the latest standards and takes due account of our corporate development. The safety and health of our employees and the public, as well as the protection of our neighbourhoods and the environment, have high priority at all Group facilities. In all we do, we are guided by the principle of sustainable development.
Download Brutrax Overview
Brutrax® (Aastrozole Tablet) may help reduce your risk of breast cancer recurrence sold by Taj Pharmaceuticals Limited (India), a global pharmaceuticals company. It is available in India, Middle East and a few other South Asian countries.
![]() Patient Counseling Information PDF
Patient Counseling Information PDF
![]() Full Prescribing Information PDF
Full Prescribing Information PDF
Note: This product information is intended only for residents of the India. Taj Pharmaceuticals Limited, medicines help to treat and prevent a range of conditions—from the most common to the most challenging—for people around the world. The Price of the drugs indicated above may not match the actual price at which they are sold. Prices can change depending on many factors, including local taxes. These are only approximate indicative prices of the drug. The products discussed herein may have different product labelling in different countries. The product information provided in this site is intended only for the residents of India.
Information for Health Care Professionals
*** Please consult local Prescribing Information for any product before use. This website is an international information resource for healthcare professionals with an interest in disease management. This website is not intended to replace the advice of a qualified healthcare professional. Above brand is a trademark of the Taj group of companies (Taj Pharmaceuticals Limited).
Taj Pharma Group
TajPharmaceuticals India

Pharmaceuticals Manufacturing Company Taj API

Active Pharmaceuticals Ingredients Taj Agro

FMCG Company India Taj Drugs

Generics Pharmaceuticasls Exporter Taj Lifesciences

Lifesciences Company Taj Hospitals

Hospitals in India Taj For Doctors

Information to Patients and Doctors




 Anastrozole 1mg Tablets.png)